S. cerevisiae VN-fusion Library Individual Strains (Agar type)
Yeast genome-wide protein-protein interaction libraries
본 제품은 형광단백질을 N-말단 및 C-말단 절편으로 나눈 후 상호작용을 알아보고자 하는 두 단백질에 부착하여 발현되게 한 다음, 두 단백질이 상호작용을 하기 위해 가까워질 경우 형광단백질의 두 절편이 합쳐져 온전한 형광단백질이 형성될 때 나타나는 형광을 분석하는 방법 (이분자 형광 상보 기법)을 이용하여 개발되었습니다. 그리하여 살아있는 세포에서 단백질 상호작용 확인이 가능하며 상호작용이 일어나는 세포 내 위치까지 분석할 수 있다는 장점을 가지고 있습니다. 현재 yeast proteome의 약 93% 확보하여 5,809개의 VN-tagged Open Reading Frames (ORFs)으로 구성되어 있으므로 실험 목적에 맞는 유전형의 균주를 각각 선택하여 실험에 사용하실 수 있습니다.
특장점
-
살아있는 세포 내에서 단백질 상호작용과 세포 내 위치 분석이 가능
-
Fluorescent complex 형성 시, 형광 signal이 발산되어 형광 현미경만으로 간단하게 관찰 가능
-
단백질의 상호 작용을 유전체적 수준에서 분석 가능 (90% 이상 coverage 가짐)
-
알려지지 않은 새로운 단백질의 기능 및 상호작용 연구에 용이
-
Proteinylation 분석 가능 (ubiquitination, sumoylation, neddylation 등)
개요
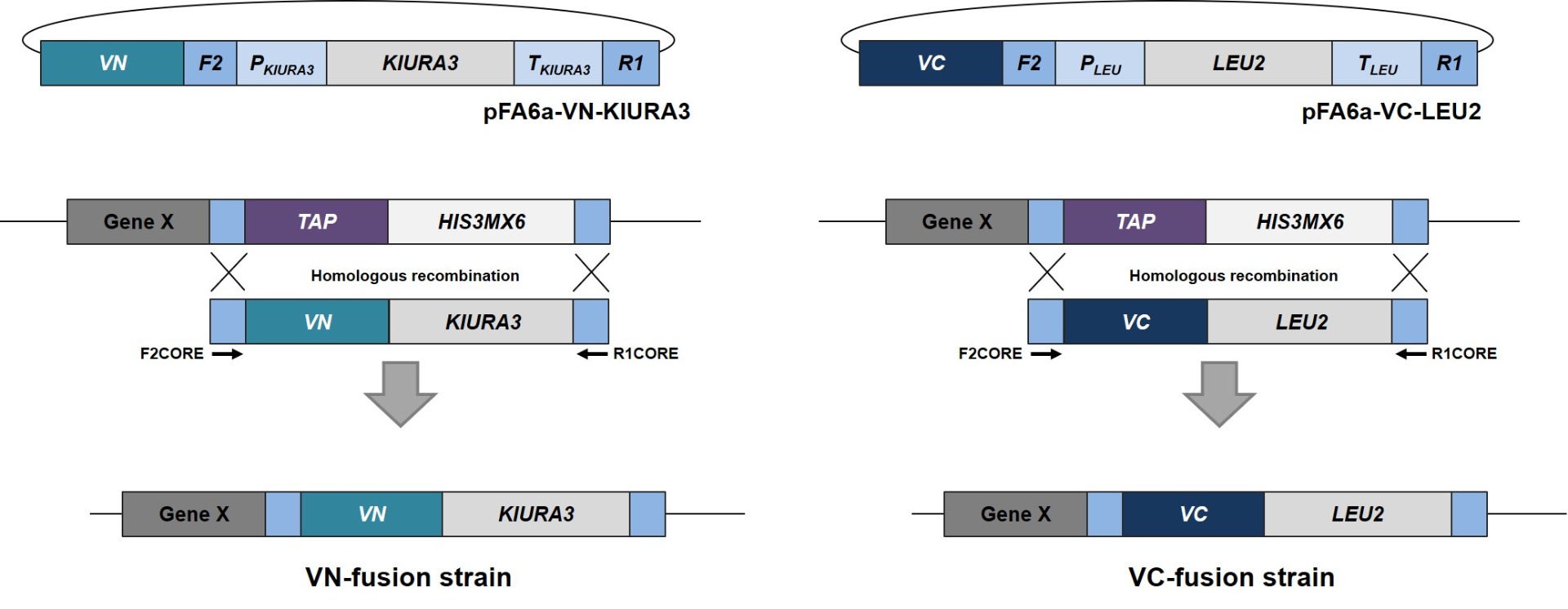
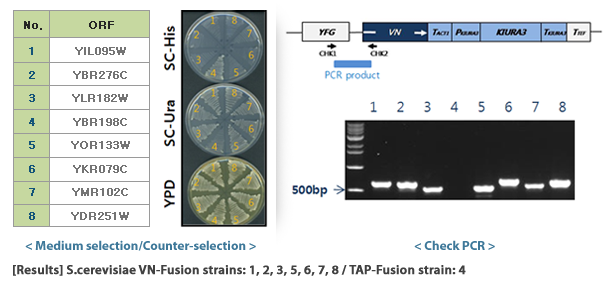
S. cerevisiae VENUS-fusion library 서울대학교에서 개발하였으며 (VN-fusion Library : Genome Res. 2013. 23:736-746, VC-fusion Library: Genome Res. 2019. 29:135-145) 바이오니아가 독점 사업권을 가지고 있습니다. VENUS-fusion library는 C-말단에 VENUS의 각 절편 (VN & VC)을 포함하는 ORF를 발현하는 S.cerevisiae 균주 library 입니다. VN/VC 융합 단백질은 homologous recombination 을 통해 yeast 염색체에 삽입되었고, 자체 promotor를 사용하여 발현됩니다. VN library는 5,809개, VC library는 5,552개의 균주로 구성되어 있어 S.cerevisiae 프로테옴의 90% 이상을 커버할 수 있습니다. .
원리
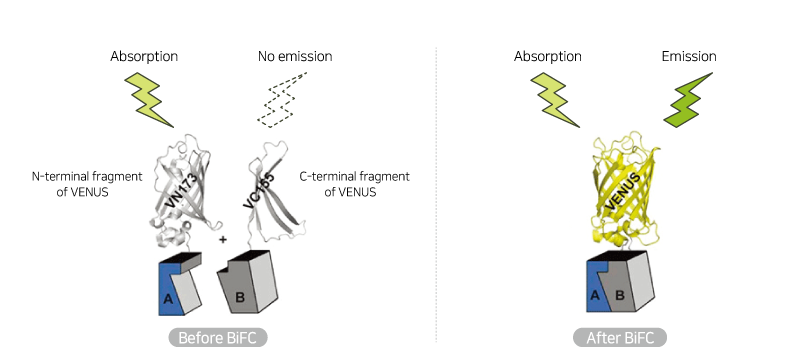
이분자 형광 상보 기법 (bimolecular fluorescence complementation; BiFC) Assay
이분자 형광 상보 기법 (bimolecular fluorescence complementation; BiFC)이란, 형광 단백질(VENUS)을 N-말단(VN) 및 C-말단(VC) 절편으로 나눈 뒤 상호작용을 알아보고자 하는 두 단백질에 각각 부착하여 발현 시킨 후, 두 단백질이 멀리 떨어져 있을 때는 형광을 내지 않다가 VENUS 형광 단백질의 두 절편이 가까워지면, 온전한 형광 단백질 복합체를 형성하여 형광을 내는 현상을 이용하는 기법입니다. 형광 신호를 현미경을 통해 바로 측정하기 때문에 단백질의 상호 작용을 연구할 때 간편하게 사용할 수 있습니다.
응용 분야
응용 분야 1. 세포 내에서 단백질-단백질 상호작용의 세포 내 위치 분석
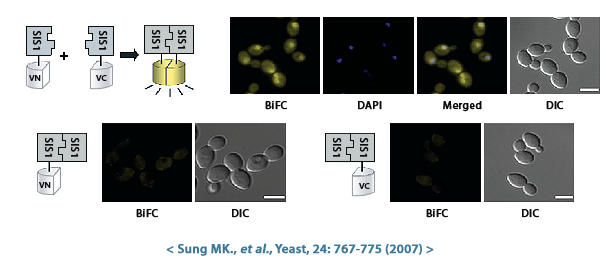
Figure 1. Homodimer를 형성하는 것으로 알려진 두 단백질(Sis1 & Ssa1)의 결합을 VN/VC-fusion library를 이용하여 확인함.
Sis1 (Type II HSP40 co-chaperone)과 Ssa1 (HSP70 protein)이 결합하면서 VENUS 형광으로 나타나며, 핵과 세포질에서 결합함을 확인할 수 있음.